Molecules of Ice Have Less Mass Than Molecules of Water
The molecules of cold water are larger than the molecules of hot water. Ice floats in water.

The Structure Of Ice Chemistry For Non Majors
No the mass is identical but the volume and thus density of a lot of molecules together differ that is.

. Molecules in the gas phase have more kinetic energy than in solids. The water molecules in ice have less mass than in liquid water b. Less density means less mass per unit volume densitymassvolume.
As in the question volume is same more density means more mass. Their atomic numberatomic mass does not change just because of temperature. Water vapor is less dense than ice because 1 molecules in the gas phase are in constant motion.
If a person compares the same amount of ice and water ice does not have more mass than liquid water. X 2 - 2x 1 -x. The water molecules are more closely packed together in ice than in water.
8 Why is ice less dense than liquid water quizlet. 1 Show answers Another question on Chemistry. However the volume of the ice is greater than that of liquid water.
Water vapor is less dense than ice because A. The bonding of liquid water involves hydrogen bonds attaching each water molecule to. Molecules of ice have less mass than molecules of water.
12 What is the density when water turns into ice. 9 How does density compared to water. Molecules in the gas phase have more potential energy than in solids.
Molecules in the gas phase are in constant motion. Username E-Mail Password Confirm Password Captcha Giải phương trình 1 ẩn. Gas microscopic-Vibrate move freely at high speed-compressible-Lots of free space between particles.
A ice molecules have more mass than water molecules B ice molecules have less mass than water molecules C a volume of ice has less mass than the same volume of water D one gram of ice has less volume than one gram of water. Ice molecules have less mass than water molecules. Depends on the kind of iceMolecules are molecules.
The average speed of the water molecules is higher but the average kinetic energy. One gram of ice has less volume than 1 g of water. The water molecules form crystalline lattice structures in ice where they are more spread out d.
Molecules in ice are more spread out than molecules in liquid water. A reason for this occurring involves the molecular structure of these two different states of water. Gaseous molecules have less mass.
A volume of ice has less mass than the same volume of water. 13 Which has more density liquid or. The water molecules in ice have less volume than in liquid ice.
2 molecules in the gas phase have more potential energy than in solids. Ice molecules have less mass than water molecules. Do molecules of ice have less mass than molecules of water.
Molecules of ice have less mass than molecules of water. Ice molecules have less mass than water molecules. Molecules of Ice and molecules of water are exactly the same.
Which must be true. But CO2 ice would indeed be different. Molecules in ice have less space between them than molecules in liquid water.
Molecules of ice have less mass than molecules of water. Hence 1 litre of water will have more mass than same amount of ice and hence more number of molecules. The molecules of cold water have more mass than the molecules of hot water.
5 molecules in the gas phase have more space between them than in solids. Ice has less density than water as it floats over water. Gaseous molecules have less mass.
The molecules of cold water are closer together than the molecules of hot water. Hỏi x. Do molecules of ice have less mass than a molecule of water.
At any given temperature how does the behavior of the two compare. Ice molecules have more mass than water molecules. Do molecules of ice have less mass than molecules of water.
Another dives 5 m in a pool. Molecules of ice have less mass than molecules of water. 4 gaseous molecules have less mass.
Water vapor is less dense than ice because Select one. Molecules in the gas phase have more space between them than in solids. 10 When water freezes its density increases or decreases.
The molecules of cold water have fewer heat molecules mixed with them. Molecules in the gas phase are in constant motion. 3 molecules in the gas phase have more kinetic energy than in solids.
One person walks 5 m down a hill. Why is ice less dense than water. No the mass is identical but the volume and thus density of a lot of molecules together differ that is.
Molecules in the gas phase have more kinetic energy than in solids. Identify a strong intermolecular force of attraction between an alcohol. Water molecules have less mass than the sodium atoms in the salt.
Ice molecules have more mass than water molecules. A Plasma is a gas with an electrical charge. 11 Why is ice less dense than water Brainly.
Why is an ice cube less dense than liquid water.
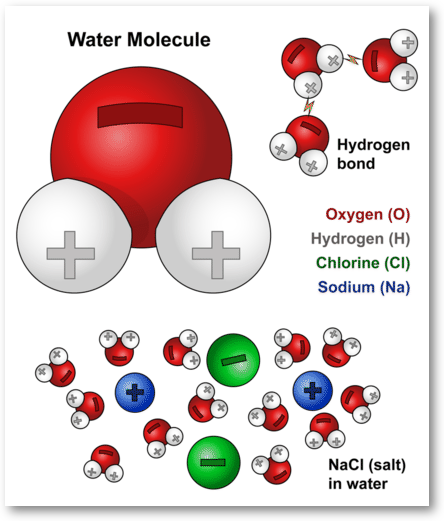
Water Molecules And Their Interaction With Salt U S Geological Survey

What Are Some Examples Of Molecules With Hydrogen Bonding Hydrogen Bond Bond Theology
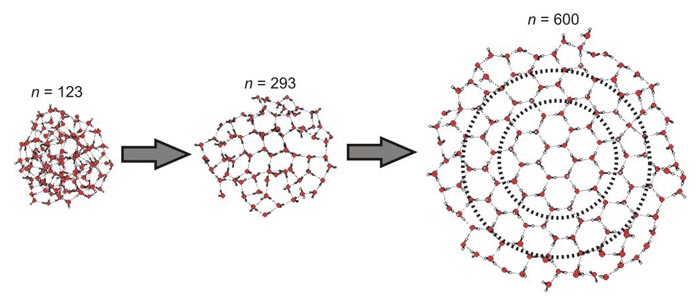
How Many Water Molecules Does It Take To Make Ice Physics World
Comments
Post a Comment